KEYNOTE-A39a: A Pivotal Phase 3 Trial for KEYTRUDA + EV in Patients With LA/mUC1
An open-label, randomized, multicenter study of KEYTRUDA + EV vs gemcitabine + cisplatin or carboplatin in patients with previously untreated LA/mUC (N=886)

At trial initiation, maintenance therapy was not an approved treatment option. Once it was approved, a protocol amendment occurred.
Maintenance therapy was then allowed, at the discretion of the investigator and where available, to be used following completion and/or discontinuation of platinum-containing therapy.1,2
KEYNOTE-A39 is also known as EV-302.2
IV infusion over 30 minutes. When administered on the same day, KEYTRUDA was given approximately 30 minutes after EV.
As assessed by BICR according to RECIST v1.1.
As assessed by BICR.
AUC = area under the curve; BICR = blinded independent central review; CNS = central nervous system; IV = intravenous; PD-L1 = programmed death ligand 1; RECIST v1.1 = Response Evaluation Criteria In Solid Tumors v1.1.
When determining a treatment option for my appropriate patients with locally advanced or metastatic urothelial cancer, I consider the patient population studied in the clinical trials. In my practice, I see patients who fit within the indicated patient population studied in KEYNOTE-A39, which helps factor into my decision-making.
Jeanny Aragon-Ching, MD, FACP, FASCO
Fairfax County, VA
Baseline Patient Characteristics

Treatments received in the chemotherapy control arm at first cycle:
- Of the 234 cisplatin-eligible patients, 220 (94.0%) received cisplatin at first cycle, 8 received carboplatin at first cycle, and 6 were never treated.
- Of the 210 cisplatin-ineligible patients, 205 (97.6%) received carboplatin at first cycle; 5 were never treated.
3 patients had an unknown primary site of disease origin.
ECOG PS = Eastern Cooperative Oncology Group performance status.
KEYNOTE-A39a: For the 1L Treatment of Adult Patients With LA/mUC2
Superior OS With KEYTRUDA + EV vs gemcitabine + cisplatin or carboplatin

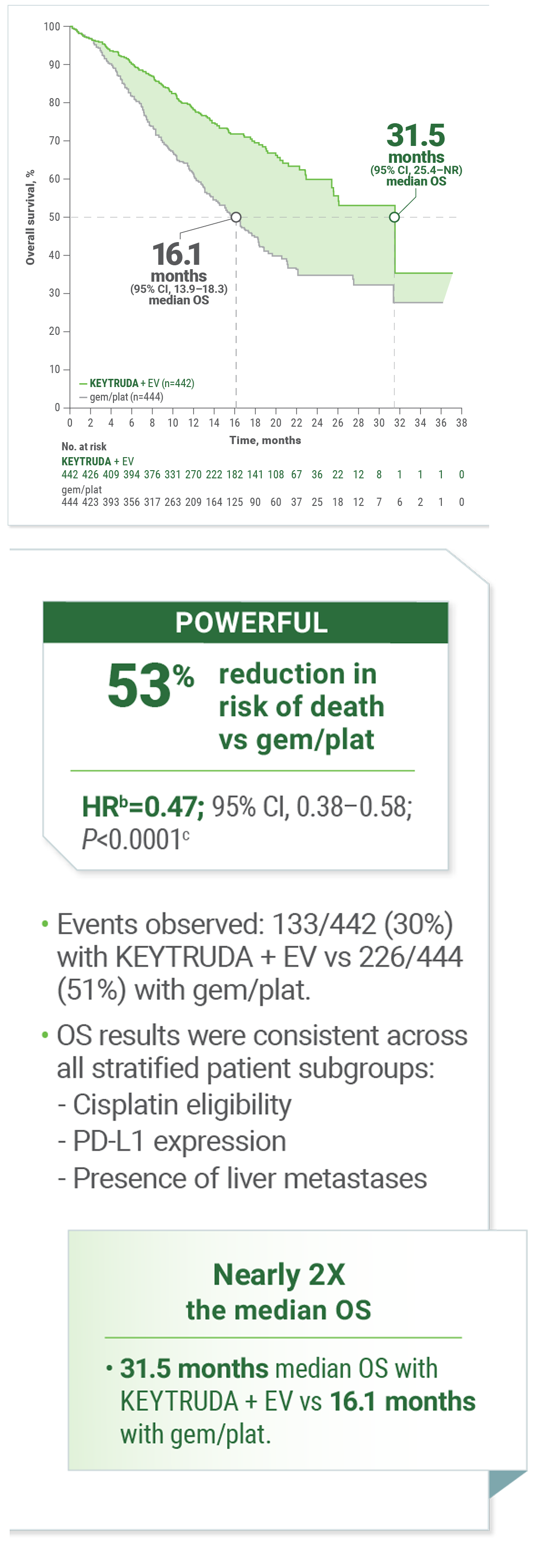
KEYNOTE-A39 is also known as EV-302.2
Calculated using stratified Cox proportional hazard regression model.
Two-sided P value based on stratified log-rank test.
CI = confidence interval; gem/plat = gemcitabine with cisplatin or carboplatin; HR = hazard ratio; NR = not reached; OS = overall survival.
When considering treatment options for my appropriate patients with locally advanced or metastatic urothelial cancer, I review the clinical findings for available treatments. I encourage you to review the efficacy and safety results from KEYNOTE-A39 to learn more about KEYTRUDA in combination with enfortumab vedotin.
Mike Lattanzi, MD
Austin, TX
KEYNOTE-A39: Exploratory Analysis of Prespecified Subgroups2,3
OS in Cisplatin-Eligible and Cisplatin-Ineligible Patients3
LIMITATIONS: In KEYNOTE-A39, formal statistical testing for OS based on cisplatin eligibility was not conducted. The study was not powered to detect differences in the treatment effect based on cisplatin eligibility. Therefore, results should be interpreted with caution and no conclusions should be drawn.

From: N Engl J Med; Powles T, Valderrama BP, Gupta S, et al. Supplementary appendix to: Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. Vol 390(10), Page 10. Copyright © 2024 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
KEYNOTE-A39: Exploratory Analysis of Prespecified Subgroups1,3
OS Across Prespecified Subgroups2
LIMITATIONS: In KEYNOTE-A39, formal statistical testing for these subgroups was not conducted. The study was not powered to detect differences in the treatment effect in these subgroups. Therefore, results should be interpreted with caution and no conclusions should be drawn.

From: N Engl J Med; Powles T, Valderrama BP, Gupta S, et al. Supplementary appendix to: Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. Vol 390(10), Page 884. Copyright © 2024 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
KEYNOTE-A39: For the 1L Treatment of Adult Patients With LA or mUC2
Superior PFS With KEYTRUDA + EV vs gemcitabine + cisplatin or carboplatin
Kaplan-Meier Estimates of PFS

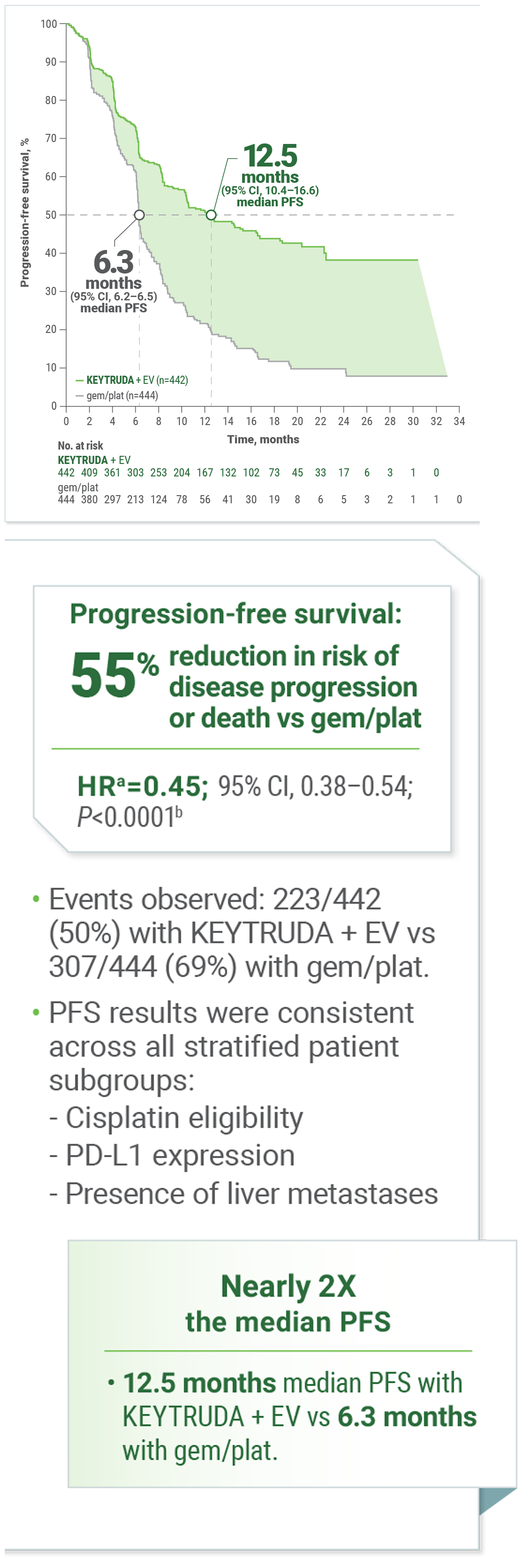
Calculated using stratified Cox proportional hazard regression model.
Two-sided P value based on stratified log-rank test.
I consider KEYTRUDA in combination with enfortumab vedotin as a potential first-line treatment option for appropriate patients with locally advanced or metastatic urothelial cancer, regardless of their cisplatin eligibility, after reviewing the efficacy and safety data from the KEYNOTE-A39 study.
Manojkumar Bupathi, MD
Littleton, CO
KEYNOTE-A39: For the 1L Treatment of Adult Patients With LA or mUC2
Superior ORR With KEYTRUDA + EV vs gemcitabine + cisplatin or carboplatin
68% of patients responded to KEYTRUDA + EV vs 44% with gem/plat

- ORR results were consistent across all stratified patient subgroups:
- Cisplatin eligibility
- PD-L1 expression
- Presence of liver metastases
Includes only patients with measurable disease at baseline (n=437 for KEYTRUDA + EV, n=441 for gem/plat) and is based on patients with a best overall response as confirmed complete or partial response.
Two-sided P value based on Cochran-Mantel-Haenszel test stratified by PD-L1 expression, cisplatin eligibility, and liver metastases.
CR = complete response; PR = partial response.
I encourage careful review of the results from KEYNOTE-A39, in which KEYTRUDA was evaluated in combination with enfortumab vedotin and demonstrated a statistically significant and superior objective response rate of 68% in the first-line treatment of patients with locally advanced or metastatic urothelial cancer, compared with 44% of patients treated with gemcitabine with cisplatin or carboplatin.
Rahul Ravilla, MD, MS
Albany, NY
KEYNOTE-A39: Selected Safety Profile
The safety of KEYTRUDA in combination with enfortumab vedotin was investigated in KEYNOTE-A39 in patients with locally advanced or metastatic urothelial cancer. A total of 440 patients received KEYTRUDA 200 mg on day 1 and enfortumab vedotin 1.25 mg/kg on days 1 and 8 of each 21-day cycle compared with 433 patients who received gemcitabine on days 1 and 8 and investigator’s choice of cisplatin or carboplatin on day 1 of each 21-day cycle.


Fatal adverse reactions
Fatal adverse reactions occurred in 3.9% of patients treated with KEYTRUDA in combination with enfortumab vedotin including acute respiratory failure (0.7%), pneumonia (0.5%), and pneumonitis/ILD (0.2%).
Serious adverse reactions
Serious adverse reactions occurred in 50% of patients receiving KEYTRUDA in combination with enfortumab vedotin. Serious adverse reactions in ≥2% of patients receiving KEYTRUDA in combination with enfortumab vedotin were rash (6%), acute kidney injury (5%), pneumonitis/ILD (4.5%), urinary tract infection (3.6%), diarrhea (3.2%), pneumonia (2.3%), pyrexia (2%), and hyperglycemia (2%).
Permanent discontinuation
Permanent discontinuation of KEYTRUDA occurred in 27% of patients. The most common adverse reactions (≥2%) resulting in permanent discontinuation of KEYTRUDA were pneumonitis/ILD (4.8%) and rash (3.4%).
Dose interruptions
Dose interruptions of KEYTRUDA occurred in 61% of patients. The most common adverse reactions (≥2%) resulting in interruption of KEYTRUDA were rash (17%), peripheral neuropathy (7%), COVID-19 (5%), diarrhea (4.3%), pneumonitis/ILD (3.6%), neutropenia (3.4%), fatigue (3%), alanine aminotransferase increased (2.7%), hyperglycemia (2.5%), pneumonia (2%), and pruritus (2%).

Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available KEYTRUDA (range: 407 to 439 patients).
Graded per NCI CTCAE v4.03.
ILD = interstitial lung disease; COVID-19 = Coronavirus Disease 2019; NCI CTCAE = National Cancer Institute Common Terminology Criteria for Adverse Events.

Clinically relevant adverse reactions (<20%) include pyrexia (18%), dry skin (17%), vomiting (12%), pneumonitis/ILD (10%), hypothyroidism (10%), blurred vision (6%), infusion site extravasation (2%), and myositis (0.5%).
Graded per NCI CTCAE v4.03.
Includes multiple terms.
KEYNOTE-A39 Dosing Schedule

Patients received enfortumab vedotin 1.25 mg/kg as an IV infusion over 30 minutes on Days 1 and 8 of a 21-day cycle followed by KEYTRUDA 200 mg as an IV infusion on Day 1 of a 21-day cycle approximately 30 minutes after enfortumab vedotin. Patients were treated until disease progression or unacceptable toxicity. In the absence of disease progression or unacceptable toxicity, KEYTRUDA was continued for up to 2 years.
IV = intravenous; Q3W = every 3 weeks.
References: 1. Powles T, Valderrama BP, Gupta S, et al. Protocol for: Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med. 2024;390(10):875-888. doi:10.1056/NEJMoa2312117 2. Powles T, Valderrama BP, Gupta S, et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med. 2024;390(10):875-888. doi:10.1056/NEJMoa2312117 3. Powles T, Valderrama BP, Gupta S, et al. Supplementary appendix to: Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med. 2024;390(10):875-888. doi:10.1056/NEJMoa2312117









